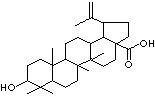
BETULINIC ACID
PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
Analgesic, Anti-Infective, Anti-Inflammatory agent, Antimalarial, Antiparasitic, Antiprotozoal, Antirheumatic, Hormone Substitute, Prostaglandin antagonist
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
Slightly soluble in petroleum ether, DMF, DMSO, benzene. Soluble in methanol, ethanol, chloroform, ether. Highly soluble in pyridine, acetic acid.
AUTOIGNITION
NFPA RATINGS
Health hazard: 0, Flammability: 0, Physical hazards: 0
REFRACTIVE INDEX
Stable under ordinary conditions
GENERAL DESCRIPTION & APPLICATIONS
Betulinic acid and its derivatives have been discovered as a new class of compounds that seem to protect the cells of human immunological system in vitro from attack by the HIV virus (Soler et al. 1996). Synthetic betulinic acid derivatives, especially 3-alkylamido-3-deoxy-betulinic acid derivatives, inhibit the life cycle of the virus in the infected cells in its early phase, and thus defend the surrounding cells from HIV proliferation (Kashiwada et al. 2000). The exact mechanism of this action still remains unclear. It is very probable that betulinic acid derivatives combine with the protein coat of the virus and thus hinder its binding to the cellular membrane of the host cell. Unbound virus is incapable of reproduction. The antiviral properties of betulinic acid were also confirmed in clinical trials (De Clercq 2000). Until now only monoclonal antibodies and peptides have been reported to inhibit HIV's attachment to the membranes of the host cell. A group of Japanese scientists has confirmed that betulinic and also some other triterpenoide acids inhibit HIV 1 replication (Maet al. 1999). In order to elucidate which structural elements were responsible for the antiviral activity, other derivatives were tested (Kanamoto et al. 2001) . Dihydrobetulinic acid was found to be the most potent HIV replication inhibitor (Kashiwada et al. 1996; Hashimoto et al. 1997).(http://www.zsf.jcu.cz/)
Betulinic acid is a pentacyclic triterpene. It has several botanical sources, but can also be chemically derived from betulin, a substance found in abundance in the outer bark of white birch trees (Betula alba). Betulinic acid has been found to selectively kill human melanoma cells while leaving healthy cells alive. For the past four decades, the incidence of melanoma has been increasing at a higher rate than any other type of cancer (source:http://biotech.icmb.utexas.edu/)Betulinic acid, a pentacyclic triterpene, is known to have inhibitory property for aminopeptidase N (EC 3.4.11.2) activity and thus is a potential anti-melanoma agent by selectively killing human melanoma cells. It is a chemical compounds that may assist in fighting oral bacteria which would promote tooth decay. It is also considered to have effective as a non-steroidal anti-inflammatory agent, antimalarial, and prostaglandin antagonist.
- Analgesic
- Anti-HIV Agent
- Anti-infective Agent
- Anti-inflammatory Agent
- Antimalarial
- Antineoplastic Agent
- Antiparasitic Agent
- Antiprotozoal Agent
- Antiretroviral Agent
- Antirheumatic Agent
- Antiviral Agent
- Hormone Antagonist
- Peripheral Nervous System Agent
- Prostaglandin antagonist
- Sensory System Agent
APPEARANCE
ASSAY (G.C)
98.0% min
OPTICAL ROTATION
+7º ~ +9º (c=0.9 in pyridine)
HAZARD OVERVIEW
May be harmful if inhaled. May cause respiratory tract irritation. May be harmful if absorbed through skin. May cause skin irritation. May cause eye irritation. May be harmful if swallowed.
RISK PHRASES
SAFETY PHRASES
24/25